Vietnam starts human trials of COVID-19 vaccine
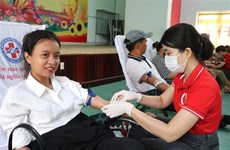
Many volunteers are students and office workers, who registered with the university to volunteer. They have already been provided with research information on the vaccine and the possible side-effects.
Nanocovax, a Made-in-Vietnam COVID-19 vaccine, will be used on humans in clinical trials starting from December 17. The company’s research and production began last March and Nanocovax was developed based on recombinant protein technology.
Volunteers will be divided into three groups, each testing a different dose of vaccine, from 25 to 50 or 75 micrograms.
With the Ministry of Health’s permission, the first phase of human trials will involve 60 people aged between 18 and 50 and in good health.
After about two to four weeks, they will be tested for COVID-19 antibodies and move to the second phase, which is set to begin in January with about 400 volunteers aged 18 to 60.
The third phase will involve 3,000 people between 12 and 70 years of age and begin in March./.