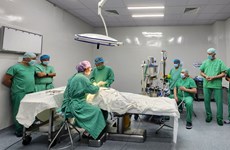
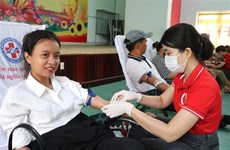
Vietnam to produce anti-H1N1 vaccine in late 2014
The Institute for Vaccines and Medical Biologicals under the Health
Ministry is stepping up preparations for production of a vaccine against
H1N1 flu virus in late 2014 or early 2015.
The Institute for Vaccines and Medical Biologicals under the Health
Ministry is stepping up preparations for production of a vaccine against
H1N1 flu virus in late 2014 or early 2015.
With approval from the Health Ministry, the institute conducted the first trial of its vaccine, A/H1N1/09, on 48 healthy people in Ben Luc district, southern Long An province from April to November 2012 to have an assessment of the vaccine’s effects and its safeness.
The trial has achieved good results as expected, so the institute expects to ask for the ministry’s permission at the end of this month to conduct the second trial on 200-300 people.
If everything continues going smoothly, the last trial will be carried out on 1,000 people before the vaccine can be produced en masse in late next year or early 2015, said Duong Huu Thai, deputy head of the institute.
Each trial will last for six months, during which every aspects of the vaccine will be examined and assessed, he added.
The price of H1N1 vaccine produced by the institute will be equal to only one-thirds of those imported, Thai said.
The H1N1 flu virus caused a world-wide pandemic in 2009. It is now a human seasonal flu virus that also circulates in pigs. The virus spreads between people in the same way that other seasonal flu viruses spread.
So far this year the virus has caused at least four deaths in Vietnam.
The institute has also been assigned by the ministry to produce another vaccine against the H5N1 avian flu virus and has received a funding of about 3 million USD from the Programme for Appropriate Technology in Health of the US Department of Health and Human Services’ Biomedical Advanced Research and Development Authority (BARDA).-VNA/Tuoitrenews
With approval from the Health Ministry, the institute conducted the first trial of its vaccine, A/H1N1/09, on 48 healthy people in Ben Luc district, southern Long An province from April to November 2012 to have an assessment of the vaccine’s effects and its safeness.
The trial has achieved good results as expected, so the institute expects to ask for the ministry’s permission at the end of this month to conduct the second trial on 200-300 people.
If everything continues going smoothly, the last trial will be carried out on 1,000 people before the vaccine can be produced en masse in late next year or early 2015, said Duong Huu Thai, deputy head of the institute.
Each trial will last for six months, during which every aspects of the vaccine will be examined and assessed, he added.
The price of H1N1 vaccine produced by the institute will be equal to only one-thirds of those imported, Thai said.
The H1N1 flu virus caused a world-wide pandemic in 2009. It is now a human seasonal flu virus that also circulates in pigs. The virus spreads between people in the same way that other seasonal flu viruses spread.
So far this year the virus has caused at least four deaths in Vietnam.
The institute has also been assigned by the ministry to produce another vaccine against the H5N1 avian flu virus and has received a funding of about 3 million USD from the Programme for Appropriate Technology in Health of the US Department of Health and Human Services’ Biomedical Advanced Research and Development Authority (BARDA).-VNA/Tuoitrenews