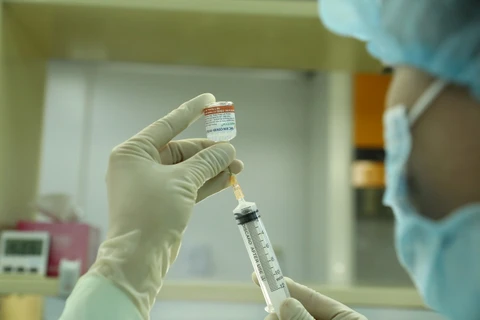
Hanoi (VNA) – A total of 367 volunteers, including 30 aged over 60, have been injected with Nano Covax, Vietnam’s first homegrown COVID-19 vaccine candidate, in the second phase of its human trials, according to the Military Medical University.
The second stage has been conducted by the university and the Ho Chi Minh City-based Pasteur Institute since February 26. It has been carried out at the university in Hanoi and the medical centre of Ben Luc district in the southern province of Long An, with the participation of 560 volunteers aged from 12-75, including 80 aged over 60.
They will receive two doses of either the vaccine or the placebo AIPO4, with an interval of 28 days. Each volunteer will be monitored for 12 months after the first dose.
Volunteers receiving shots on the morning of February 26 will receive the second doses in late March.
Lieutenant-General Do Quyet, Director of the Military Medical University, said since the trial sees the participation of volunteers with underlying health conditions, competent authorities have been prepared for all scenarios and ensure safety for all volunteers.
Results of the trial will be announced in May 2021 before preparing for the third-stage trial during which only one single shot of the vaccine will be administered to 10,000-15,000 people from both domestic and foreign pandemic-hit regions, Quyet added.
Developed by the Nanogen Pharmaceutical Biotechnology JSC and the Hanoi-based Military Medical University, Nano Covax is Vietnam’s first COVID-19 vaccine to reach the human trial stage.
The first-stage trial of the Nano Covax vaccine showed that it is likely to be effective against the B117 variant from the UK.
Vietnam is one of 40 countries and territories in the world to have begun human trials of a COVID-19 vaccine, after successfully producing coronavirus test kits early in the pandemic./.
The second stage has been conducted by the university and the Ho Chi Minh City-based Pasteur Institute since February 26. It has been carried out at the university in Hanoi and the medical centre of Ben Luc district in the southern province of Long An, with the participation of 560 volunteers aged from 12-75, including 80 aged over 60.
They will receive two doses of either the vaccine or the placebo AIPO4, with an interval of 28 days. Each volunteer will be monitored for 12 months after the first dose.
Volunteers receiving shots on the morning of February 26 will receive the second doses in late March.
Lieutenant-General Do Quyet, Director of the Military Medical University, said since the trial sees the participation of volunteers with underlying health conditions, competent authorities have been prepared for all scenarios and ensure safety for all volunteers.
Results of the trial will be announced in May 2021 before preparing for the third-stage trial during which only one single shot of the vaccine will be administered to 10,000-15,000 people from both domestic and foreign pandemic-hit regions, Quyet added.
Developed by the Nanogen Pharmaceutical Biotechnology JSC and the Hanoi-based Military Medical University, Nano Covax is Vietnam’s first COVID-19 vaccine to reach the human trial stage.
The first-stage trial of the Nano Covax vaccine showed that it is likely to be effective against the B117 variant from the UK.
Vietnam is one of 40 countries and territories in the world to have begun human trials of a COVID-19 vaccine, after successfully producing coronavirus test kits early in the pandemic./.
VNA