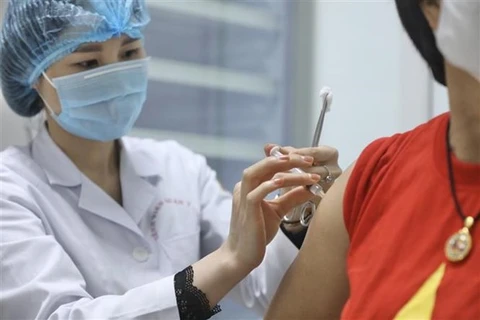
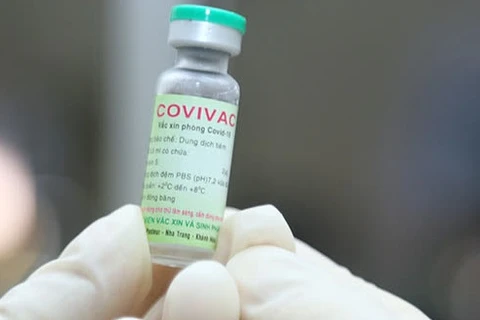
Hanoi (VNA) – Deputy Health Minister Tran Van Thuan has said his ministry supports the expansion of areas eligible for the third phase of clinical trials of Nanocovax, a product of Nanogen Pharmaceutical Biotechnology JSC based on recombinant DNA/protein technology.
The work must ensure safety and feasibility, he said.
Earlier, the vaccine underwent the first-phase test from December 18, 2020, the second phase from February 26, 2021 and the third phase from June 11, 2021.
According to the approved plan, the third-phase trials aim to assess the vaccine’s efficacy and is carried out in many centres worldwide with 13,000 volunteers.
Earlier, Deputy Prime Minister Pham Minh Chinh asked the Health Minister to direct the use and licensing of Nanocovax with less administrative procedures and in line with regulations.
At a nationwide teleconference between the Government and businesses, ministries, agencies and localities, Chinh underlined three major contents of vaccine strategy, including importing as many as vaccines possible at the earliest.
Amid the global shortage of vaccines, the country strives to access them via different channels, including stepping up vaccine diplomacy, he said./.