Hanoi (VNA) - On September 26 and 27, Takeda, a global biopharmaceutical company, hosted a series of scientific symposia in Ho Chi Minh City (HCMC) and Hanoi, in collaboration with the Pasteur Institute in Ho Chi Minh City and the Vietnam Association of Prevention Medicine (VAPM) in Hanoi.
The event themed "Vaccine: A New Paradigm in Dengue Fever Prevention in Vietnam," aimed to provide a platform for advancing knowledge sharing, fostering collaboration in holistic dengue management strategies, and introducing a new dengue vaccine available in public and private institutions.
The dengue vaccine, which was approved by the Drug Administration of Vietnam (DAV) in May 2024, will play an essential role in an integrated dengue prevention strategy in Vietnam and globally.
The symposia across two cities were attended by nearly 1,000 scientists, healthcare professionals (HCPs), along with representatives from the Vietnam Ministry of Health, Cities and provincial departments of health, National Institutes, hospitals, provincial CDCs, World Health Organisation (WHO), US CDC, International NGO, Embassy of Japan and the Japan External Trade Organization, as well as the representatives from academia, medical associations, institutes, and dengue experts in clinical and immunisation settings and public and private vaccination centers across the country

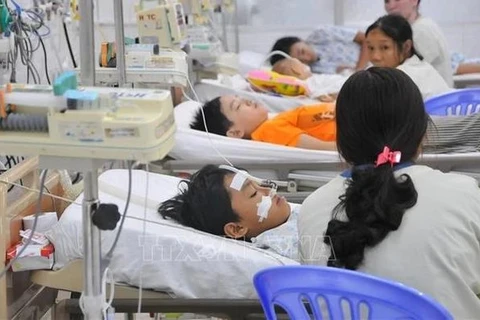
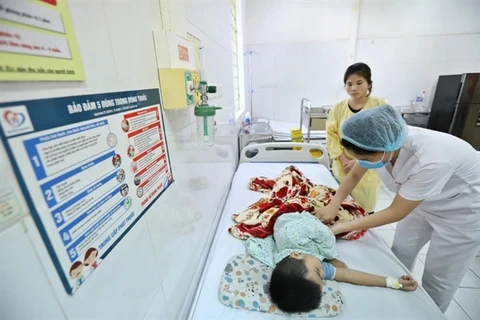
Dengue fever is among the most common mosquito-borne viral diseases worldwide and is recognised as one of the top threats to public health by the WHO. Dengue is currently endemic in more than 100 countries and causes an estimated 390 million infections each year with global incidence rates increasing 30-fold over the last 50 years due to climate change, rapid urbanization and movements of people and goods. Vietnam is listed among the top countries with highest serious burden from dengue fever by WHO. Experts warn that dengue fever is evolving to become more unpredictable and dangerous as it no longer follows the same patterns while expanding endemic areas. Previously, in the period of 1980 - 2018, Vietnam often recorded epidemic peaks every 10 years, then in the period of 2019 - 2023 alone, Vietnam experienced two epidemic peaks in 2019 (with over 300,000 cases) and 2022 (361,813 cases).
Assoc. Prof. Nguyen Vu Trung, PhD., MD., Director of the Pasteur Institute in HCMC and Chairman of the symposium in HCMC emphasised, “The peak of the dengue fever epidemic in Vietnam typically occurs in the rainy season, from July to November[1]. Recently, Vietnam records new cases of dengue fever, including severe and life-threatening cases, even during the remaining months of the year. Moreover, dengue fever typically affected the Central and Southern regions, but in recent years dengue fever has become more prevalent in the North of Vietnam. These concerning shifts have intensified the need for more immediate actions and resources within healthcare sector and community in dengue prevention and management.”
As Co-chairman of the symposium in Hanoi, Prof. Phan Trong Lan – Director of the National Institute of Hygiene and Epidemiology, Vietnam stated, “In the recent years, the Vietnamese government, healthcare agencies and the Vietnamese people have worked together to achieve progress in dengue prevention and management, especially in reducing death rates. The introduction and availability of the dengue vaccine, alongside ongoing efforts in vector control, personal protection, and community engagement, marks a significant step forward for Vietnam. These integrated dengue prevention measures will help reduce burdens on the healthcare system ultimately benefiting the country's overall health and economy”.
Prof. Nguyen Van Kinh, Ph.D., M.D. – Permanent Vice President of Vietnam Medical Association (VMA), Co-chairman of the symposium in Hanoi, said “Dengue fever not only carries the risk of severe complications, such as organ failure or death, but it also places a heavy burden on both individuals and public health systems. The disease's unpredictable progression and the absence of specific antiviral treatments make it exceptionally challenging to manage. This underscores the critical need for a strong prevention strategy to effectively reduce the overall impact of dengue.”
The symposia also featured Prof. Veerachai Wattanaveeradej, Director of Vaccine Research Center at Bangkok Phramongkutklao Hospital and Executive Committee at The Royal College Pediatricians and Pediatric Society of Thailand, who shared key learnings about the safety profile and post dengue vaccination surveillance, from clinical trials to real-life practice in Thailand – one of the top 30 countries in the world experiencing high burdens from dengue fever.
Dr. Derek Wallace, President of Global Vaccine Business Unit at Takeda, addressed the Vietnamese healthcare community on the introduction of the innovative dengue vaccine as part of a comprehensive strategy for dengue fever prevention and control. Takeda’s dengue vaccine has been recommended by WHO’s Strategic Advisory Group of Experts (SAGE) for introduction in countries with high dengue burden and high transmission intensity to maximize public health impact.

Dr. Derek Wallace shared, “While current prevention efforts are essential, the introduction of a vaccine that provides broad protection marks a significant breakthrough in the fight against this disease. Takeda’s dengue vaccine represents a major step forward in our mission to develop innovative vaccines that address the toughest public health challenges. The recent recommendation by WHO underscores the vaccine’s potential as an important tool within an integrated strategy to help reduce the global threat of dengue.”
Dion Warren, Area Head of India and Southeast Asia at Takeda, shared, “Collaboration is key in the fight against dengue. Takeda is proud to join forces with health authorities, healthcare professionals, medical societies, academia, and other partners to combat this public health threat in Vietnam. With dengue posing substantial health risks in Vietnam, the introduction of the dengue vaccine as an additional preventive tool alongside existing measures offers renewed hope. We are optimistic about the future, where integrated protection against dengue could improve the lives of countless people in Vietnam and beyond.”

In recognition of the efforts in dengue prevention and management by Vietnamese healthcare professionals, in the frame of the symposia, the photo exhibition sessions themed “Three-Decade Journey of Preventing and Controlling Dengue Fever in Vietnam” were conducted to pay tribute and honor the country’s healthcare sector and affirmed Takeda’s commitment to accompany Vietnam in a new chapter of dengue management. In HCMC, the exhibition was hosted by Assoc. Prof. Nguyen Thi Kim Tien, former Minister of Health, and in Hanoi, by Prof. Vu Sinh Nam, Senior Advisor on Dengue Fever at NIHE and Chief of Staff at VAPM.
Takeda’s dengue vaccine is currently approved for children and adults in more than 40 countries, including the European Union, Brazil, Argentina, Columbia, Indonesia, Thailand, Malaysia and Vietnam. The vaccine is accessible through some public vaccination programs in Indonesia, Brazil and Argentina. In addition to being recommended in the WHO’s dengue vaccines position paper, the vaccine has recently been included in the WHO’s List of Prequalified Vaccines, underscoring its quality and reliability as an important dengue prevention method suitable for public programmes.

Takeda’s dengue vaccine is based on a live-attenuated dengue serotype 2 virus, which provides the genetic "backbone" for all four dengue virus serotypes and is designed to protect against any of these serotypes. The vaccine should be administered subcutaneously as a 0.5 mL dose at a two-dose (0 and 3 months) schedule according to the approved dosing regimen. In Vietnam, Takeda’s dengue vaccine is approved by the Drug Administration of Vietnam (DAV) for use in individuals from age 4 and above, regardless of previous dengue exposure, mirroring the approval from the European Commission./.