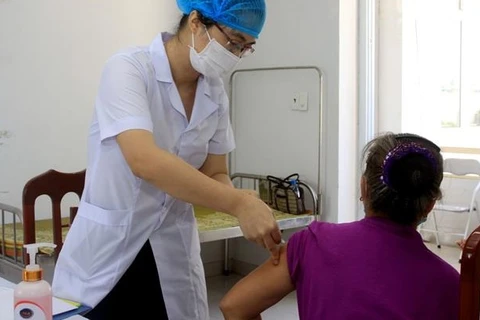
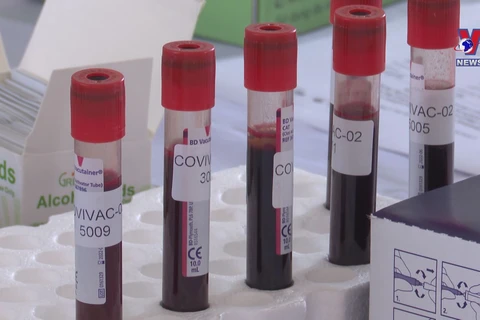
Do Minh Sy, R&D Director of the vaccine producer - Nanogen Pharmaceutical Biotechnology JSC, briefs the participants on the research and development of NanoCovax vaccine. (Photo: VNA)
Do Minh Sy, R&D Director of the vaccine producer - Nanogen Pharmaceutical Biotechnology JSC, briefs the participants on the research and development of NanoCovax vaccine. (Photo: VNA) Do Minh Sy, R&D Director of the vaccine producer - Nanogen Pharmaceutical Biotechnology JSC, briefed the participants on the research and development of NanoCovax vaccine.
Trial results have proven the safety of the vaccine, he said, adding that most of the volunteers displayed minor vaccine reactions or even no side effects.
The participants said vaccination is the quickest and safest way to reach herd immunity, and suggested relevant units focus on the trial and assessment of NanoCovax with the engagement of experts.
They also looked into COVID-19 vaccine mixing and booster shot drives launched by a number of countries given the spread of the Delta variant.
In his remarks, Deputy Minister of Foreign Affairs Pham Quang Hieu, head of the State Committee for Overseas Vietnamese Affairs, said the opinions will be collected and reported to the Government working group on COVID-19 vaccine diplomacy, and forwarded to specialised agencies for reference.
The official expressed his hope that with the leadership of the Party and the Government, and contributions of experts at home and abroad, Vietnam will successfully produce COVID-19 vaccines and effectively roll out its vaccination drive./.
VNA