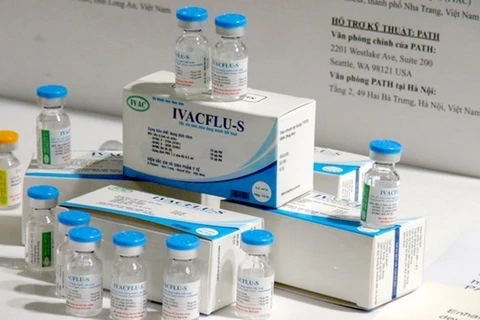
The IVACFLU-S vaccines are displayed at the Institute of Vaccines and Medical Biologicals under the Ministry of Health. (Photo: VNA)
The IVACFLU-S vaccines are displayed at the Institute of Vaccines and Medical Biologicals under the Ministry of Health. (Photo: VNA) Hanoi (VNA) - The IVACFLU-S vaccines, which have been produced in Vietnam to protect against seasonal flu such as A/H1N1, A/H3N2, and B, are now available on the market, according to the Institute of Vaccines and Medical Biologicals under the Ministry of Health.
As many as 50,000 doses of vaccines have been produced for the first time at the cost of between 120,000-180,000 VND (5.2-7.8 USD) per dose, half the cost of imported vaccines.
The influenza vaccines have been studied and produced based on embryonic egg production technology in 2010 under co-operation between the institute and the Program for Appropriate Technology in Health (PATH).
The institute had undergone multiple phases of clinical trials from 2017 onward. It has proved to be safe and capable of strengthening adults’ immune systems.
On January 14, 2019, the vaccines were licensed by the Ministry of Health to protect against seasonal flu including H1N1, H3N2 and B.
The institute is capable of manufacturing 1.5 million doses of vaccines per year.
Dr Duong Huu Thai, Director of the institute, said the vaccines have been shown as safe to improve the immune systems of people aged between 18 and 60.
Vaccines will be available in Hai Phong, Khanh Hoa, Long An, and Dong Nai provinces before being supplied to other localities.
The IVACFLU-S vaccines are contraindicated for people with a history of hypersensitivity to the ingredients in the vaccine or allergic to proteins in chicken eggs and chicken.
Seasonal flu is a respiratory disease that can lead to fatalities. As many as 650,000 deaths and about 3-5 million serious cases are recorded due to seasonal flu around the world each year.-VNA
VNA