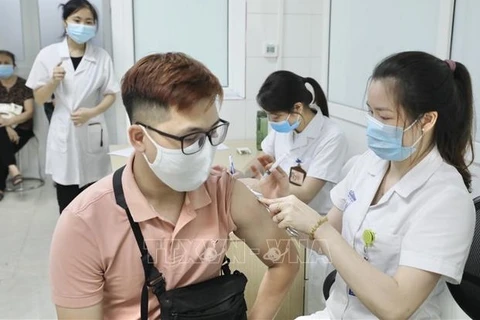
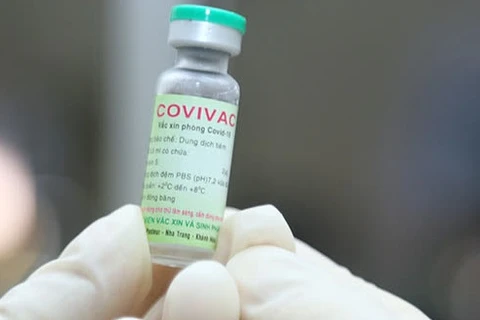
A volunteer received a shot of Nano Covax vaccine in the third phase of clinical trials on July 2 (Photo: VNA)
A volunteer received a shot of Nano Covax vaccine in the third phase of clinical trials on July 2 (Photo: VNA) The Ministry of Health and the Ministry of Science and Technology held a meeting on July 22 to review the outcomes of clinical trials of the vaccine, which is produced by Nanogen Pharmaceutical Biotechnology JSC based on recombinant DNA/protein technology.
Based on the evaluation, the vaccine may be approved for emergency use.
It went through the first phase trial from December 18, 2020, and the second phase from February 26, 2021. The third phase started on June 11, 2021.
Results from the first two trial phases showed that all volunteers developed antibodies against SARS-CoV-2.
At the meeting, a representative from Nanogen said more than 13,620 volunteers had been vaccinated with Nano Covax vaccine so far.
The third and the final phase saw participation of 13,000 volunteers with 1,004 people getting two jabs.
The company said it would continue to complete documents relating to the products and outcomes of the trials to submit to the Ministry of Health next week.
Chairman of the Advisory Council for the Registration of Circulation of Drugs and Medicinal Ingredients Le Van Truyen said the council supported the research and development of homegrown vaccines but registration dossiers for approving vaccines in emergency situations must meet scientific and legal standards.
Deputy Minister of Science and Technology Bui The Duy said under the current complex development of the pandemic, the ministry hoped health experts would evaluate outcomes of the clinical trials scientifically and create conditions to speed up research and production of the vaccine.
Deputy Minister of Health Tran Van Thuan said the ministry supported and created conditions for Nanogen as well as other domestic companies and units to research and develop vaccines with the hope that Vietnam would soon be able to produce “Made in Vietnam” vaccines to help ensure vaccine supply for coronavirus control.
Regarding licensing conditions, Thuan asked Nanogen to work with agencies to complete dossiers about the outcomes of the first and second phases of human trials and soon update results of the third phase.
Based on the outcomes of trials and the opinions of domestic and foreign experts as well as the pandemic situation and vaccine demand, the Ministry of Health will study and consider the licensing of the Nano Covax vaccine for emergency use.
Nano Covax is the most promising candidate among four domestic vaccines being developed by Vietnam. If approved, commercial production could start soon.
The other vaccines are being developed by the Institute of Vaccines and Medical Biologicals, the Vaccine and Biological Production Company No. 1, and the Center for Research and Production of Vaccines and Biologicals.
Vietnam has so far approved six vaccines for emergency use, namely Janssen, Moderna, Sputnik V, Pfizer, Sinopharm and AstraZeneca.
The Government is making efforts to secure at least 150 million vaccine doses to inoculate 70 percent of the population.
As of July 23 morning, 4,411,659 doses of the vaccines, mainly AstraZeneca, have been administered in Vietnam, with 334,560 people having received the full two doses./.
VNA