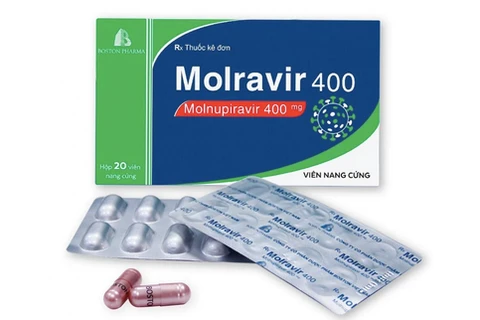
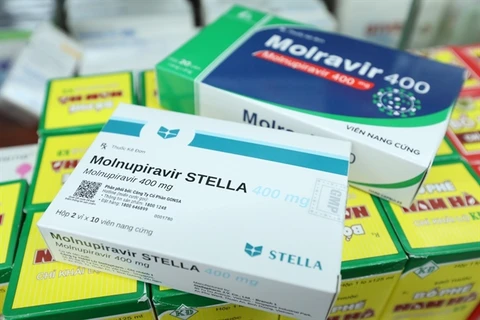
There are now four Molnupiravir-based drugs produced in Vietnam approved for domestic use. (Photo: suckhoedoisong.vn)
There are now four Molnupiravir-based drugs produced in Vietnam approved for domestic use. (Photo: suckhoedoisong.vn) This is the fourth Molnupiravir-based drug produced in Vietnam to be authorised for use in the country.
The DAV orders manufacturers to adhere to documents registered with the Ministry of Health; coordinate with treatment facilities to strictly comply with current regulations on prescription drugs, monitor the safety, effectiveness, and unwanted effects of drugs on Vietnamese people, as well as compile reports according to regulations.
At the same time, the DAV also requested local departments of health notify medical examination and treatment facilities, medical staff, and drug suppliers in the area to inform patients of the benefits and risks when using Molnupiravir, including treatments, as well as alternatives for Molnupiravir in the treatment of COVID-19.
In addition, it requires units to strengthen monitoring, supervision and detection of cases of adverse drug reactions (if any), to send reports to the National Center for Drug Information and Adverse Drug Reaction Monitoring (Hanoi) or Regional Center for Drug Information and Adverse Drug reaction monitoring (Ho Chi Minh City), according to regulations.
The DAV orders the National Center for Drug Information and Adverse Drug Reactions Monitoring-Hanoi University of Pharmacy, the Regional Center for Drug Information and Adverse Drug Reactions Monitoring in HCM City-Cho Ray Hospital, and the Center for Clinical Pharmacology-Hanoi Medical University to be responsible for monitoring, updating and reporting the following information to the Department:
- Information in the leaflets for drugs containing Molnupiravir approved in reference countries around the world to update the unified instruction sheet for Molnupiravir drugs.
- Information related to the safety and effectiveness of drugs containing Molnupiravir evaluated by pharmaceutical regulatory authorities in the world, World Health Organisation and information on the use and circulation status of drugs containing Molnupiravir worldwide.
- Information related to drug ADR reports and information on the safety and efficacy of drugs circulating in Vietnam.
The decision of the Drug Administration also states that in the course of drug circulation, based on monitoring and updating information on the safety and effectiveness of the three drugs above, the Drug Administration of Vietnam can revoke the drug registration under the provisions of Clause 1, Article 58 of the Law on Pharmacy./.
VNA