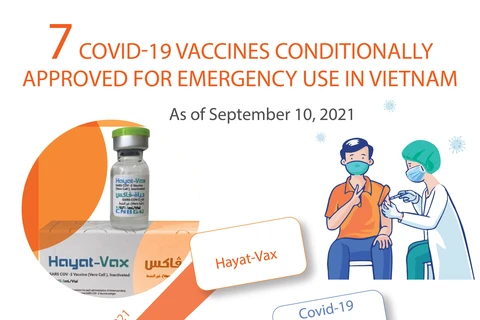
The Vietnamese Ministry of Health conditionally approved the vaccine for emergency use in Vietnam on June 28, 2021. (Photo: AFP/VNA)
The Vietnamese Ministry of Health conditionally approved the vaccine for emergency use in Vietnam on June 28, 2021. (Photo: AFP/VNA) According to a notice issued by the DAV on March 2, the expiry date of the vaccine has been extended from seven to nine months (from the manufacturing date) at the storage condition of -25 to -15 degrees Celsius, applicable to vaccine manufacturing establishments authorised by the Ministry of Health for emergency use.
The decision was made based on the conclusion of the ministry’s Advisory Council for the Registration of Circulation of Drugs and Medicinal Ingredients, the MoH said on March 3, affirming that the adjustment will not affect the quality, safety and efficiency of the vaccine.
Earlier, the extension of the shelf life of the vaccine was approved by the World Health Organisation (WHO) on February 9, 2022, the European Medicines Agency (EMA) on December 8, 2021, the US Food and Drug Administration (US FDA) on January 31, 2022, and drug agencies of the UK, Australia, Canada and Switzerland.
The Vietnamese Ministry of Health conditionally approved the vaccine for emergency use in Vietnam on June 28, 2021./.
VNA