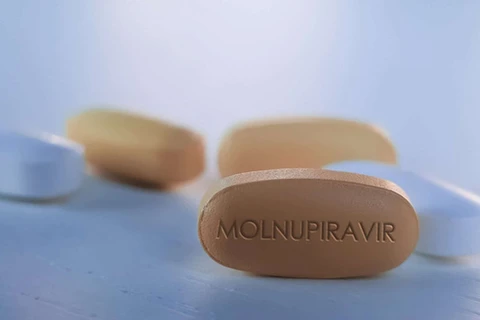
Hanoi (VNA) – An advisory council of the Ministry of Health (MoH) has proposed this ministry grant certificates of registration for conditional circulation of three medicines containing the active ingredient molnupiravir for COVID-19 treatment.
The MoH said on January 5 that the council decided to submit the proposal after making thorough and careful consideration of opinions from specialised units and experts.
To strictly control medicine quality, the council requested that manufacturers examine material quality before production; continue monitoring and examining drug quality on a monthly basis after receiving the certificates and present reports to relevant authorised agencies; and continue studying the medicines’ stability and submit research findings and expiry date updates to serve assessment as in accordance with the ASEAN Common Technical Dossier.
Regarding the authorisation of COVID-19 medicine circulation, the Minister of Health asked managerial and specialised agencies to keep a strict control of quality and prices as in line with the Law on Pharmacy and to fight against any negative phenomenon and group interest in medicine supply./.
The MoH said on January 5 that the council decided to submit the proposal after making thorough and careful consideration of opinions from specialised units and experts.
To strictly control medicine quality, the council requested that manufacturers examine material quality before production; continue monitoring and examining drug quality on a monthly basis after receiving the certificates and present reports to relevant authorised agencies; and continue studying the medicines’ stability and submit research findings and expiry date updates to serve assessment as in accordance with the ASEAN Common Technical Dossier.
Regarding the authorisation of COVID-19 medicine circulation, the Minister of Health asked managerial and specialised agencies to keep a strict control of quality and prices as in line with the Law on Pharmacy and to fight against any negative phenomenon and group interest in medicine supply./.
VNA