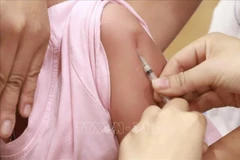
An area where volunteers will be monitored after they receive shots at the Vietnam Military Medical University (Photo: VNA)
An area where volunteers will be monitored after they receive shots at the Vietnam Military Medical University (Photo: VNA) As many as 560 volunteers, aged from 18 to 65, willparticipate in the second phase, according to Nguyen Ngo Quang, Deputy Directorof the Department of Science, Technology and Training under the Ministry ofHealth.
The testing will be carried out at the VietnamMilitary Medical University in Hanoi and in the Mekong Delta province of Long An by the HCM City-based Pasteur Institute.
Unlike the first phase when volunteers got the dosesof 25mcg, 50mcg and 75mcg, this phase’s volunteers will be injected with only50mcg and 75mcg doses, according to the health official.
Most of the volunteers of the first phase that begun onDecember 17, 2020 are in stable conditions after vaccination. Only few reported pain at the injection spot and fever that disappeared after one to two days.
Developed by the Nanogen Pharmaceutical BiotechnologyJSC, Nano Covax is the first Vietnam-made COVID-19 vaccine to enter humantrials, with another two from other manufacturers to follow in February andMarch.
Vietnam is one of the 40 countries that have conductedhuman trials of a COVID-19 vaccine.
The country also has several other COVID-19 candidatevaccines being developed, by the Institute of Vaccines and Medical Biologicals(IVAC), the Company for Vaccine and Biological Production No.1 (VABIOTECH), andthe Centre for Research and Production of Vaccines and Biologicals (POLYVAC)./.