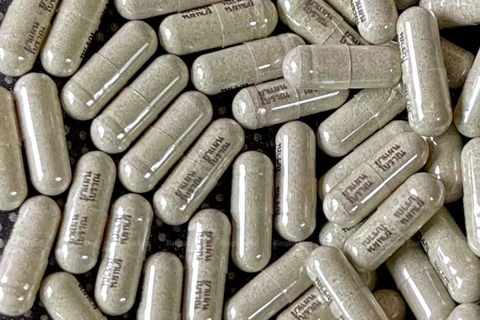
Bangkok (VNA) – Thailand’s Food and Drug Administration (FDA) has approved the use of Favipiravir tablets manufactured by the Government Pharmaceutical Organisation (GPO) in Thailand for the treatment of COVID-19 to meet rising demand in the country.
GPO expert Nantakarn Suwanpitakkun said made-in-Thailand Favipiravir tablets cost only half the prices of their imported equivalent, while their quality is the same.
Thailand needs about 300,000 Favipiravir tablets each day, or about 9 million a month. Earlier, GPO had imported an additional 5.5 million tablets for treatment after the third wave of COVID-19 starting in early April quickly spread to many countries.
In recent days, the number of daily new COVID-19 cases in Thailand continued to surge to new records. On July 25 morning, the health ministry announced 15,335 cases in the past 24 hours, raising the national tally to 497,302. The same day, the pandemic claimed 129 more lives, bringing the total fatalities to 4,059.
Thailand has so far administered 15.3 million doses of COVID-19 vaccine./.