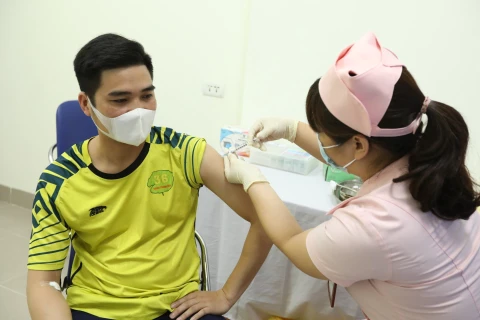
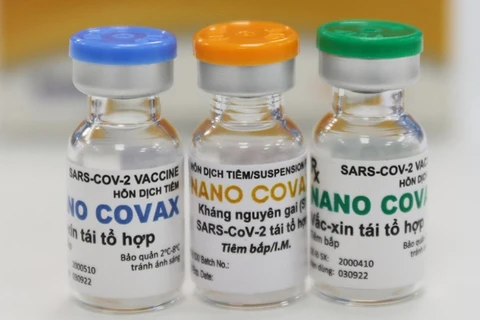
Nano Covax vaccine is researched and developed by the Nanogen Pharmaceutical Biotechnology JSC (Photo: VNA)
Nano Covax vaccine is researched and developed by the Nanogen Pharmaceutical Biotechnology JSC (Photo: VNA) If the trial goes well, in the context of vaccine scarcity the Ministry of Health will submit to competent agencies for approval of the use of homegrown vaccines in emergency circumstances.
Several companies have invested in plants and are seeking vaccine production technologies both in and outside the country. At least one large-scale COVID-19 vaccine production plant is scheduled to be put in operation in late 2021 or early 2022.
The Ministry of Health has urged the Company for Vaccine and Biological Production No.1 (VABIOTECH) to conduct procedures to receive Sputnik V vaccine production technology from Russia in two phases. In the first phase, the company will import materials from Russia for bottling and production is expected to begin in July.
The Ministry of Health will coordinate with ministries and sectors to submit to the Government a proposal on accessing all vaccine supply sources in the world towards the goal of achieving herd immunity by the end of 2021, and create mechanisms to facilitate investors’ investment in the production of vaccines in the country to serve not only domestic use but also export./.
VNA