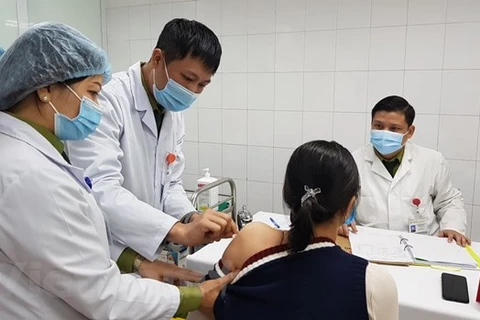
COVIVAC vaccine developed by the Institute of Vaccines and Biological Medical (IVAC) (Photo: VietnamPlus)
COVIVAC vaccine developed by the Institute of Vaccines and Biological Medical (IVAC) (Photo: VietnamPlus) Hanoi (VNA) – Vietnam has contained the spread of COVID-19 over the recent past and is among a few countries making strides in vaccine development.
A ceremony was held at the Hanoi Medical University (HMU) on January 21 to kick-start clinical trials of COVIVAC, Vietnam’s second COVID-19 vaccine candidate and developed based on the new highly-infectious coronavirus variants.
It applies primary chicken embryo cell culture, a technique used previously to successfully produce seasonal flu vaccines and shipped to many other countries in the world.
Pre-clinical period proved that COVIVAC has many advantages, including high immunogenicity and suitability for infrastructure at vaccination facilities in Vietnam, as well as effectiveness against SARS-CoV-2 variants, said Dr Pham Van Tac, Director of the Ministry of Health’s Administration of Science Technology and Training during a launch ceremony for clinical trial of COVIVAC held by the Institute of Vaccines and Biological Medical (IVAC).
Strengths of COVIVAC
COVIVAC has undergone pre-clinical trials in India, the US, and Vietnam, said IVAC Director Dr Duong Huu Thai, adding that results showed that it satisfies all conditions to be tested on humans.
The vaccine candidate is being developed by IVAC and universities, research institutes and international organisations.
It does not contain adjuvants and conservatives, and is developed with a technology of Vietnam in vaccine production which tackles seasonal influenza, Thai explained.
He added that with completed and concerted infrastructure, 6 million doses of COVIVAC may be manufactured a year and the amount could hit 30 million. The institute may produce about 500,000 doses per batch.
Of especial note, it only requires to be stored at 2-8 degree Celcius, while Pfizer/BioNTech vaccine must be kept at minus 70 degree Celcius and can only be used within five days at room temperature.
Clinical testing on more than 120 people
Prof. Dr. Ta Thanh Van, Rector of the Hanoi Medical University (HMU), said that plans for phases 1 and 2 of clinical trials of the vaccine have been assessed by the ethics councils in relevant agencies.
The trials will be carried out by the National Institute of Hygiene and Epidemiology together with the HMU. A center for clinical pharmacology at the university, recognised as meeting Good Clinical Research Practice (GCP), will be responsible for the process under the supervision of the Health Ministry.
The first phase is expected to select 120 volunteers with good health. The volunteers, aged 18 to 59, has been recruited by the research team. Previously, Nano Covax was tested on 60 volunteers in its first phase.
Volunteers for COVIVAC will be divided into five groups, comprising of three without adjuvants with three doses of 1 mcg, 3 mcg and 10 mcg, one group of 1-mcg dose with adjuvants and another of placebo.
Volunteers will receive two shots (vaccine or placebo) and the second jab will be 28 days after the first one.
The first shot is scheduled to be injected at the Hanoi Medical University in February.
If data on health conditions of volunteers on their 43rd day shows that the vaccine fulfils safety criteria and produces antibodies effective in COVID-19 prevention, the second phase will be rolled out with a larger number of volunteers.
In last December, the Health Ministry, the Military Medical University under the Defence Ministry, and Nanogen Pharmaceutical Biotechnology JSC officially launch a project on clinical trials of Nano Covax developed by Nanogen.
Twenty volunteers received the second shots of Nanocovax on January 20.
Out of over 500 applicants for the vaccine trials, more than 200 underwent health check-ups, of whom 51 people are eligible for the first stage.
The second stage, starting from February, is expected to conduct trials on 400-600 people aged between 12-17.
If successful, Vietnam will begin mass production of the COVID-19 vaccine in early 2022./.