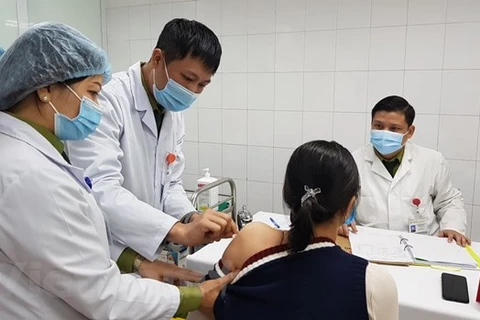
The first human trial of COVID-19 vaccine Nanocovax begins in Vietnam on December 17 with the first three volunteers, receiving injections at the Military Medical Academy. (Photo: VNA)
The first human trial of COVID-19 vaccine Nanocovax begins in Vietnam on December 17 with the first three volunteers, receiving injections at the Military Medical Academy. (Photo: VNA) Hanoi (VNA) – The trials of COVID-19 vaccine in Vietnam strictly follow the guidance of the World Health Organisation (WHO) and international organisations in terms of safety and immunity, said Foreign Ministry’s Spokesperson Le Thi Thu Hang at a press conference in Hanoi on January 14.
She said that the Military Medical University gave 75mcg of Nanocovax, a COVID-19 vaccine developed by Vietnam, to three volunteers on January 12. This is the highest dose of the vaccine given to volunteers during the first stage of human trials.
Nanocovax is developed by the Nanogen Pharmaceutical Biotechnology JSC, basing on recombinant protein technology. Currently, there has been no evidence that new COVID-19 variants can resist this technology./.
VNA