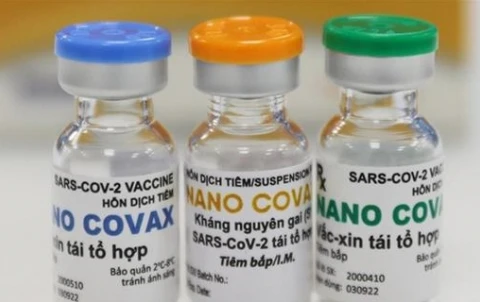
Hanoi (VNA) - The National Ethics Committee in Biomedical Research under the Ministry of Health on September 19 issued a press statement on its conclusions of a meeting one day earlier during which it looked into the mid-term phase 3 trial’s results of homegrown COVID-19 vaccine Nano Covax.
The data available for review is from the developers – Nanogen Pharmaceutical Biotechnology JSC in Ho Chi Minh City with the support in administration and medical monitoring from the Military Medical University in Hanoi – up until September 2 this year, as the final phase of clinical trials is ongoing for the recombinant DNA vaccine.
Regarding the vaccine safety, Nano Covax is deemed to be safe in the short term based on reports of phase 3 trials (during the monitoring of the volunteers’ health seven days after administration of the first dose for 11,430 volunteers, seven days after the administration of second doses for 5,785 volunteers).
Regarding immunogenicity, Nano Covax is deemed to be able to elicit immune responses, based on ongoing phase 3 results – anti-SARS-CoV-2 IgG test results on 924 samples 42 days after first jab; results of neutralising antibodies tests on 761 samples 42 days after first jab; plaque-reduction neutralisation test 42 days after the first jab on 107 samples with the first detected strain of coronavirus, 41 samples with Delta variant, 39 samples with Alpha variant.
The council’s statement stressed that to date they haven’t had the data to “directly" evaluate the protective efficacy, or the ability of a vaccine to prevent infection or symptomatic infections (based on of the number of people infected with COVID-19 after getting the shots), and said more study is needed for assessment of this "most important criteria to determine the vaccine's quality."
The currently available estimation of Nano Covax’s protective efficacy extrapolated from its immunogenicity, or the level of antibodies in the vaccinated, however, has “enough scientific grounds” for the council to send the vaccine documents to the Advisory Council for the Registration of Circulation of Drugs and Medicinal Ingredients for reviews.
Regarding the proposal to grant Nano Covax conditional approval for emergency use, the ethics committee said they reached ‘consensus’ in the use of mid-term phase 3 trials for the drugs advisory body to deliberate.
But it asked the vaccine candidate’s developers to supplement their reports as per the conclusions of the meeting and continue with the trials of the vaccine based on the approved outline to complete trials in March 2022, and give timely updates to the relevant committees and health authorities.
Experts from the Advisory Council for the Registration of Circulation of Drugs and Medicinal Ingredients, who will take up the case and make decisions on whether to give the emergency approval for the vaccine to be used in Vietnam, were also present at the meeting, the press statement reads.
Currently, 13,000 volunteers in phase 3 trials of Nano Covax – which started on July and is set to wrap up by 2022 February – have been given the second shots of the vaccine with dosage set at 25mcg.
Under the latest guidelines from the health ministry, domestically developed COVID-19 vaccines could be authorised for emergency use if they prove to be safe and effective in mid-term phase 3 findings, with further monitoring required even after obtaining such approval./.
The data available for review is from the developers – Nanogen Pharmaceutical Biotechnology JSC in Ho Chi Minh City with the support in administration and medical monitoring from the Military Medical University in Hanoi – up until September 2 this year, as the final phase of clinical trials is ongoing for the recombinant DNA vaccine.
Regarding the vaccine safety, Nano Covax is deemed to be safe in the short term based on reports of phase 3 trials (during the monitoring of the volunteers’ health seven days after administration of the first dose for 11,430 volunteers, seven days after the administration of second doses for 5,785 volunteers).
Regarding immunogenicity, Nano Covax is deemed to be able to elicit immune responses, based on ongoing phase 3 results – anti-SARS-CoV-2 IgG test results on 924 samples 42 days after first jab; results of neutralising antibodies tests on 761 samples 42 days after first jab; plaque-reduction neutralisation test 42 days after the first jab on 107 samples with the first detected strain of coronavirus, 41 samples with Delta variant, 39 samples with Alpha variant.
The council’s statement stressed that to date they haven’t had the data to “directly" evaluate the protective efficacy, or the ability of a vaccine to prevent infection or symptomatic infections (based on of the number of people infected with COVID-19 after getting the shots), and said more study is needed for assessment of this "most important criteria to determine the vaccine's quality."
The currently available estimation of Nano Covax’s protective efficacy extrapolated from its immunogenicity, or the level of antibodies in the vaccinated, however, has “enough scientific grounds” for the council to send the vaccine documents to the Advisory Council for the Registration of Circulation of Drugs and Medicinal Ingredients for reviews.
Regarding the proposal to grant Nano Covax conditional approval for emergency use, the ethics committee said they reached ‘consensus’ in the use of mid-term phase 3 trials for the drugs advisory body to deliberate.
But it asked the vaccine candidate’s developers to supplement their reports as per the conclusions of the meeting and continue with the trials of the vaccine based on the approved outline to complete trials in March 2022, and give timely updates to the relevant committees and health authorities.
Experts from the Advisory Council for the Registration of Circulation of Drugs and Medicinal Ingredients, who will take up the case and make decisions on whether to give the emergency approval for the vaccine to be used in Vietnam, were also present at the meeting, the press statement reads.
Currently, 13,000 volunteers in phase 3 trials of Nano Covax – which started on July and is set to wrap up by 2022 February – have been given the second shots of the vaccine with dosage set at 25mcg.
Under the latest guidelines from the health ministry, domestically developed COVID-19 vaccines could be authorised for emergency use if they prove to be safe and effective in mid-term phase 3 findings, with further monitoring required even after obtaining such approval./.
VNA