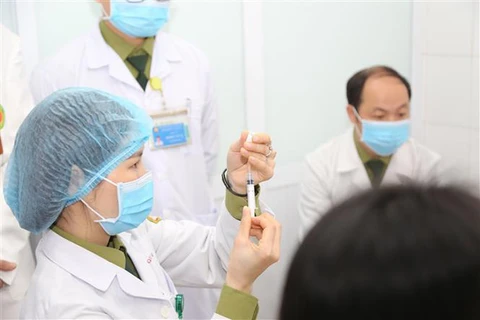
Hanoi (VNA) – More than 6,000 volunteers have registered for the third phase of human trials of homegrown Nano Covax vaccine, which is scheduled to begin on June 8, according to the Military Medical University (MMU) under the Defence Ministry.
A MMU representative said in this phase, the vaccine, developed by Vietnamese Nanogen Pharmaceutical Biotechnology JSC, will be tested on around 13,000 volunteers aged between 18 and 74, in many localities nationwide.
The third phase, also the final one, aims to evaluate the effectiveness of the vaccine and each volunteer may only receive one jab of 25 mcg.
Director of the university Prof. Dr. Do Quyet showed his confidence in the safety of Nano Covax vaccine with the positive results obtained from the previous two trial phases.
Navo Covax vaccine started testing on humans on December 17 last year and has completed the second phase.
The first-phase trials showed the vaccine was safe and vaccinated volunteers had antibodies against the UK variant (B117).
The second-phase ones were conducted on 560 volunteers divided into four groups, with 80 people injected with a placebo and three other groups administered with 25mcg, 50mcg, and 75mcg doses. However, only 554 received second shots because six people withdrew from the trial.
All of the vaccinated volunteers have developed antibodies against COVID-19 at different levels.
Regarding the antibody index to neutralise the virus, people injected with 25mcg dose got the highest index with more than 90 percent at 14 days after the second shot and 42 days since the first jabs./.
A MMU representative said in this phase, the vaccine, developed by Vietnamese Nanogen Pharmaceutical Biotechnology JSC, will be tested on around 13,000 volunteers aged between 18 and 74, in many localities nationwide.
The third phase, also the final one, aims to evaluate the effectiveness of the vaccine and each volunteer may only receive one jab of 25 mcg.
Director of the university Prof. Dr. Do Quyet showed his confidence in the safety of Nano Covax vaccine with the positive results obtained from the previous two trial phases.
Navo Covax vaccine started testing on humans on December 17 last year and has completed the second phase.
The first-phase trials showed the vaccine was safe and vaccinated volunteers had antibodies against the UK variant (B117).
The second-phase ones were conducted on 560 volunteers divided into four groups, with 80 people injected with a placebo and three other groups administered with 25mcg, 50mcg, and 75mcg doses. However, only 554 received second shots because six people withdrew from the trial.
All of the vaccinated volunteers have developed antibodies against COVID-19 at different levels.
Regarding the antibody index to neutralise the virus, people injected with 25mcg dose got the highest index with more than 90 percent at 14 days after the second shot and 42 days since the first jabs./.
VNA