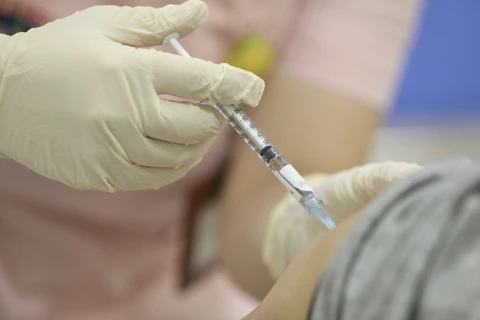
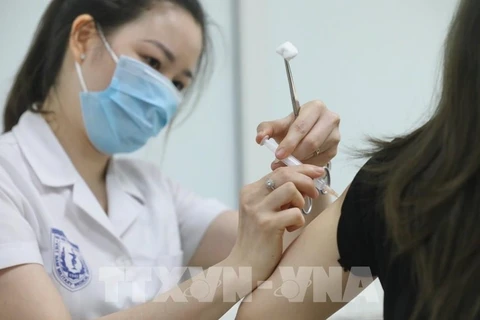
A medical worker gives a shot of Nano Covax to a volunteer in the third-phase trials at the Hanoi-based Vietnam Military Medical University (Photo: VNA)
A medical worker gives a shot of Nano Covax to a volunteer in the third-phase trials at the Hanoi-based Vietnam Military Medical University (Photo: VNA) Nguyen Ngo Quang, Deputy Director of the Health Ministry’s Department for Science, Technology and Training, made the remark in response to the Nanogen Pharmaceutical Biotechnology JSC’s proposal for validating its Nano Covax, a COVID-19 vaccine candidate, for emergency use.
Quang noted that before approving a vaccine for mass use, it is a principle that the Ministry of Health (MoH) must ensure the safety, the generation of immunity, and the protective effect of that vaccine.
The emergency use authorisation for a vaccine depends on many factors, and the MoH needs scientific data to make a decision, Quang emphasised.
He stressed the MoH’s absolute support for the development of domestic vaccines, adding that the health sector’s ultimate goal is to protect people’s health, so benefits and risks must be thoroughly considered.
Nano Covax is currently in the third phase of trials, with 1,000 volunteers already getting the shots.
Results recorded so far showed that this vaccine candidate is safe and has generated the immune response in volunteers, but its protective effect is still under research.
To have sufficient scientific basis to assess the immunity generation and protective effect, volunteers need to be monitored for 36 days, 45 days, and 56 days since the first jabs, according to the official./.
VNA