According to Le Hoang Nam, Director of the provincial PreventiveHealthcare Centre, after the Government approved the resumption of thevaccine’s use earlier this month, the centre has organised trainingcourses on vaccination procedures and safety for staff in 161 approvedhealthcare centres. More than 500 local health workers have receivedcertificates after joining the courses, he added.
The preparatory works in vaccination centres have been carried outcarefully to make sure children and their parents get healthconsultations, pre-vaccination checks and close supervision during andafter the vaccination, and ensure hygiene as well as the quality of thevaccine.
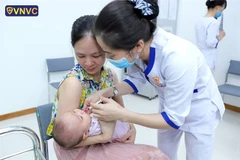
The use of Quinvaxem has resumed after afive-month suspension following the death of nine children who receivedthe vaccine earlier this year.
The vaccine,which prevents five common, potentially fatal childhood diseases,including diphtheria, tetanus, pertussis, hepatitis B and pneumonia, andmeningitis, was tested by the Ministry of Health and other domesticand international organisations. Findings failed to provide evidencethat the deaths were related to the quality of the vaccine, expertssaid.
The vaccine, manufactured by the Republicof Korea’s Berna Biotec Company and imported by the ministry, has beenused in the country since 2010.-VNA