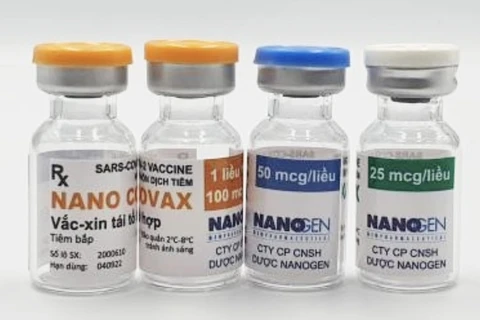
Hanoi (VNA) – More than 900 out of 12,000 volunteers participating in the second stage of the third phase of the homegrown COVID-19 vaccine Nano Covax’s clinical trial received their first shots on July 2.
The second stage is being carried out by the Military Medical University and the Health Department of Hung Yen province in the northern region, and the Pasteur Institute in Ho Chi Minh City and the Preventive Medicine Centres of Long An and Tien Giang provinces in the southern region.
All the volunteers are expected to have received their first jabs by July 15 and the second shots by August 15.
The health of 1,000 volunteers who were inoculated in the first stage of the third phase is stable. They are expected to get their second jabs on July 10.
The volunteers will have blood samples taken to test the generation of immunity by July 30, and data about the vaccine safety and immunity generation are set to be available by mid-September.
The third-phase trials, using only the 25mcg doses, cover 13,000 volunteers aged between 18 and 75 nationwide.
Results of the first two stages show good immunity generation in all the volunteers.
Nano Covax has been developed by the Nanogen Pharmaceutical Biotechnology JSC since May 2020. The first-phase trials began on December 18, 2020 while the second phase on February 26 this year, and the third on June 11.
Vietnam also has several other candidate vaccines under development./.
The second stage is being carried out by the Military Medical University and the Health Department of Hung Yen province in the northern region, and the Pasteur Institute in Ho Chi Minh City and the Preventive Medicine Centres of Long An and Tien Giang provinces in the southern region.
All the volunteers are expected to have received their first jabs by July 15 and the second shots by August 15.
The health of 1,000 volunteers who were inoculated in the first stage of the third phase is stable. They are expected to get their second jabs on July 10.
The volunteers will have blood samples taken to test the generation of immunity by July 30, and data about the vaccine safety and immunity generation are set to be available by mid-September.
The third-phase trials, using only the 25mcg doses, cover 13,000 volunteers aged between 18 and 75 nationwide.
Results of the first two stages show good immunity generation in all the volunteers.
Nano Covax has been developed by the Nanogen Pharmaceutical Biotechnology JSC since May 2020. The first-phase trials began on December 18, 2020 while the second phase on February 26 this year, and the third on June 11.
Vietnam also has several other candidate vaccines under development./.
VNA