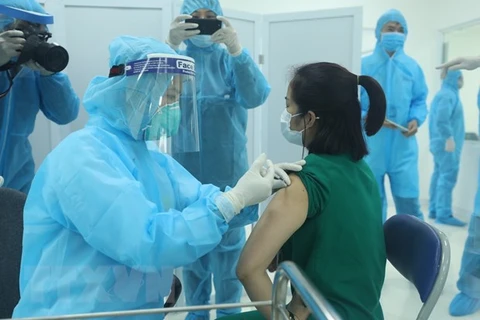
The area for vaccination services at the National Hospital for Tropical Diseases No. 2 in Hanoi’s suburb district of Dong Anh. Vietnam launches its COVID-19 inoculation drive on March 8 morning, administering the AstraZeneca vaccine to medical workers in the capital city of Hanoi, Ho Chi Minh City and the northern province of Hai Duong – the country’s biggest pandemic hotspot at present. The Hanoi hospital is among four health care facilities joining the launch, besides the HCM City Hospital for Tropical Diseases and two medical centres in Hai Duong. Vaccination priority will be given to frontliners in the fight against the virus. (Photo: VietnamPlus)
The area for vaccination services at the National Hospital for Tropical Diseases No. 2 in Hanoi’s suburb district of Dong Anh. Vietnam launches its COVID-19 inoculation drive on March 8 morning, administering the AstraZeneca vaccine to medical workers in the capital city of Hanoi, Ho Chi Minh City and the northern province of Hai Duong – the country’s biggest pandemic hotspot at present. The Hanoi hospital is among four health care facilities joining the launch, besides the HCM City Hospital for Tropical Diseases and two medical centres in Hai Duong. Vaccination priority will be given to frontliners in the fight against the virus. (Photo: VietnamPlus)  A refrigerated truck of the Vietnam Vaccine JSC (VNVC) arrives in the National Hospital for Tropical Diseases’ Dong Anh campus in Hanoi, carrying the first batch of COVID-19 AstraZeneca vaccine it received from the Ministry of Health. The hospital has been allocated with 450 doses, out of 117,600 doses commercially brought from AstraZeneca which were delivered to Vietnam in the first shipment on February 2. The vaccine order costs over 12 billion VND (520,000 USD), with import fee waived and VAT of only 5 percent. As many as 100 staff members of the hospital are expected to be inoculated in the morning of March 8. (Photo: VietnamPlus)
A refrigerated truck of the Vietnam Vaccine JSC (VNVC) arrives in the National Hospital for Tropical Diseases’ Dong Anh campus in Hanoi, carrying the first batch of COVID-19 AstraZeneca vaccine it received from the Ministry of Health. The hospital has been allocated with 450 doses, out of 117,600 doses commercially brought from AstraZeneca which were delivered to Vietnam in the first shipment on February 2. The vaccine order costs over 12 billion VND (520,000 USD), with import fee waived and VAT of only 5 percent. As many as 100 staff members of the hospital are expected to be inoculated in the morning of March 8. (Photo: VietnamPlus)  A total of 420 medical staff of the hospital will be injected with the AstraZeneca vaccine, according to Doctor Vu Minh Dien, deputy director of the National Hospital for Tropical Diseases’ centre for epidemic prevention and vaccination. Developed by Oxford University and AstraZeneca, the vaccine can prevent people from becoming ill from COVID-19 by stimulating the body’s natural immune system. It causes the body to produce its own protection – antibodies – against the virus. It is recommended for adults aged 18 years and older. The initial course consists of two doses with an interval of 8 to 12 weeks between doses for optimal efficacy, according to the World Health Organisation (WHO). (Photo: VietnamPlus)
A total of 420 medical staff of the hospital will be injected with the AstraZeneca vaccine, according to Doctor Vu Minh Dien, deputy director of the National Hospital for Tropical Diseases’ centre for epidemic prevention and vaccination. Developed by Oxford University and AstraZeneca, the vaccine can prevent people from becoming ill from COVID-19 by stimulating the body’s natural immune system. It causes the body to produce its own protection – antibodies – against the virus. It is recommended for adults aged 18 years and older. The initial course consists of two doses with an interval of 8 to 12 weeks between doses for optimal efficacy, according to the World Health Organisation (WHO). (Photo: VietnamPlus)  Some 420 staff members of the National Hospital for Tropical Diseases who provide direct care and treatment for patients with COVID-19 or are highly exposed to the virus are in line to receive the first jab of the AstraZeneca vaccine. The hospital has been allocated with 450 doses, out of 117,600 doses commercially brought from AstraZeneca which were delivered to Vietnam in the first shipment in February. Developed by Oxford University and AstraZeneca, the vaccine can prevent people from becoming ill from COVID-19 by stimulating the body’s natural immune system. It causes the body to produce its own protection – antibodies – against the virus. (Photo: VietnamPlus)
Some 420 staff members of the National Hospital for Tropical Diseases who provide direct care and treatment for patients with COVID-19 or are highly exposed to the virus are in line to receive the first jab of the AstraZeneca vaccine. The hospital has been allocated with 450 doses, out of 117,600 doses commercially brought from AstraZeneca which were delivered to Vietnam in the first shipment in February. Developed by Oxford University and AstraZeneca, the vaccine can prevent people from becoming ill from COVID-19 by stimulating the body’s natural immune system. It causes the body to produce its own protection – antibodies – against the virus. (Photo: VietnamPlus)  Three vaccination units are set up at the hospital’s centre for epidemic prevention and vaccination. VNVC has signed a contract with AstraZeneca last November to procure a total of 30 million doses that will be delivered in phases throughout 2021. The vaccine has been shown to be well-tolerated and effective for preventing symptomatic COVID-19. A single dose of the vaccine has an efficacy of 76 percent against symptomatic COVID-19 in the first 90 days after vaccination, with no significant waning of protection during this period. Vaccine efficacy after the second dose was higher in those with a longer interval, reaching 81.3 percent in those with a dosing interval of 12 weeks or more. (Photo: VietnamPlus)
Three vaccination units are set up at the hospital’s centre for epidemic prevention and vaccination. VNVC has signed a contract with AstraZeneca last November to procure a total of 30 million doses that will be delivered in phases throughout 2021. The vaccine has been shown to be well-tolerated and effective for preventing symptomatic COVID-19. A single dose of the vaccine has an efficacy of 76 percent against symptomatic COVID-19 in the first 90 days after vaccination, with no significant waning of protection during this period. Vaccine efficacy after the second dose was higher in those with a longer interval, reaching 81.3 percent in those with a dosing interval of 12 weeks or more. (Photo: VietnamPlus)  VNVC has signed a contract with AstraZeneca last November to procure a total of 30 million doses that will be delivered in phases throughout 2021. The first shipment of 117 doses arrived in Vietnam on February 24 and was transported to a specialised cold chain storage facility run by VNVC. This is a part of the initial order 204,000 which was given import emergency approval by the Ministry of Health, after a surge in community outbreaks in the last 27 days. This is in addition to about 30 million doses of Oxford/AstraZeneca vaccines that will be made available to the country via the WHO-led Vaccines Global Access (COVAX) initiative. (Photo: VietnamPlus)
VNVC has signed a contract with AstraZeneca last November to procure a total of 30 million doses that will be delivered in phases throughout 2021. The first shipment of 117 doses arrived in Vietnam on February 24 and was transported to a specialised cold chain storage facility run by VNVC. This is a part of the initial order 204,000 which was given import emergency approval by the Ministry of Health, after a surge in community outbreaks in the last 27 days. This is in addition to about 30 million doses of Oxford/AstraZeneca vaccines that will be made available to the country via the WHO-led Vaccines Global Access (COVAX) initiative. (Photo: VietnamPlus)  AstraZeneca’s COVID-19 vaccine has been authorised for emergency use by the WHO for active immunisation to prevent COVID-19 in individuals 18 years of age and older earlier this year. AstraZeneca has been working with the vaccine-sharing scheme COVAX to begin supplying the vaccine around the world, with the majority going to low and middle-income countries as quickly as possible. In the first half of 2021, it is hoped that more than 300 million doses of the vaccine will be made available to 145 countries through COVAX, pending supply and operational challenges. These doses will be allocated equitably according to the COVAX Allocation Framework. (Photo: VietnamPlus)
AstraZeneca’s COVID-19 vaccine has been authorised for emergency use by the WHO for active immunisation to prevent COVID-19 in individuals 18 years of age and older earlier this year. AstraZeneca has been working with the vaccine-sharing scheme COVAX to begin supplying the vaccine around the world, with the majority going to low and middle-income countries as quickly as possible. In the first half of 2021, it is hoped that more than 300 million doses of the vaccine will be made available to 145 countries through COVAX, pending supply and operational challenges. These doses will be allocated equitably according to the COVAX Allocation Framework. (Photo: VietnamPlus)  AstraZeneca was the first global pharmaceutical company to join COVAX in June 2020. This global mechanism is working to accelerate the development, production and equitable access to new COVID-19 tools across the world for all participating countries, regardless of income level. AstraZeneca has committed to making its COVID-19 vaccine available to as many countries as possible and at no profit during the pandemic period. Its COVID-19 vaccine has been authorised for emergency use by the WHO earlier this year. The vaccine can be stored, transported and handled at normal refrigerated conditions (2 – 8 degrees Celsius) for at least six months and administered within existing healthcare settings. (Photo: VietnamPlus)
AstraZeneca was the first global pharmaceutical company to join COVAX in June 2020. This global mechanism is working to accelerate the development, production and equitable access to new COVID-19 tools across the world for all participating countries, regardless of income level. AstraZeneca has committed to making its COVID-19 vaccine available to as many countries as possible and at no profit during the pandemic period. Its COVID-19 vaccine has been authorised for emergency use by the WHO earlier this year. The vaccine can be stored, transported and handled at normal refrigerated conditions (2 – 8 degrees Celsius) for at least six months and administered within existing healthcare settings. (Photo: VietnamPlus)  AstraZeneca’s COVID-19 vaccine uses a replication-deficient chimpanzee viral vector based on a weakened version of a common cold virus (adenovirus) that causes infections in chimpanzees and contains the genetic material of the SARS-CoV-2 virus spike protein. After vaccination, the surface spike protein is produced, priming the immune system to attack the SARS-CoV-2 virus if it later infects the body. The WHO emergency use approval was based on pooled analysis for efficacy from 11,636 participants aged 18 years and older, accruing 131 symptomatic COVID-19 infections from the UK and Brazil Phase-3 trials conducted by Oxford University. AstraZeneca has committed to making its COVID-19 vaccine available to as many countries as possible and at no profit during the pandemic period. (Photo: VietnamPlus)
AstraZeneca’s COVID-19 vaccine uses a replication-deficient chimpanzee viral vector based on a weakened version of a common cold virus (adenovirus) that causes infections in chimpanzees and contains the genetic material of the SARS-CoV-2 virus spike protein. After vaccination, the surface spike protein is produced, priming the immune system to attack the SARS-CoV-2 virus if it later infects the body. The WHO emergency use approval was based on pooled analysis for efficacy from 11,636 participants aged 18 years and older, accruing 131 symptomatic COVID-19 infections from the UK and Brazil Phase-3 trials conducted by Oxford University. AstraZeneca has committed to making its COVID-19 vaccine available to as many countries as possible and at no profit during the pandemic period. (Photo: VietnamPlus)  AstraZeneca’s COVID-19 vaccine has been authorised for emergency use by the WHO for active immunisation to prevent COVID-19 in individuals 18 years of age and older earlier this year. The vaccine has been granted a conditional marketing authorisation or emergency use in more than 50 countries, with the WHO Emergency Use Listing (EUL) accelerating the pathway to access in up to 145 countries through the COVAX Facility. AstraZeneca is a global, science-led biopharmaceutical company that focuses on the discovery, development and commercialisation of prescription medicines. Based in Cambridge, the UK, AstraZeneca operates in over 100 countries and its innovative medicines are used by millions of patients worldwide. (Photo: VietnamPlus)
AstraZeneca’s COVID-19 vaccine has been authorised for emergency use by the WHO for active immunisation to prevent COVID-19 in individuals 18 years of age and older earlier this year. The vaccine has been granted a conditional marketing authorisation or emergency use in more than 50 countries, with the WHO Emergency Use Listing (EUL) accelerating the pathway to access in up to 145 countries through the COVAX Facility. AstraZeneca is a global, science-led biopharmaceutical company that focuses on the discovery, development and commercialisation of prescription medicines. Based in Cambridge, the UK, AstraZeneca operates in over 100 countries and its innovative medicines are used by millions of patients worldwide. (Photo: VietnamPlus)  AstraZeneca has been working with the vaccine-sharing scheme COVAX to begin supplying the vaccine around the world, with the majority going to low and middle-income countries as quickly as possible. In the first half of 2021, it is hoped that more than 300 million doses of the vaccine will be made available to 145 countries through COVAX, pending supply and operational challenges. These doses will be allocated equitably according to the COVAX Allocation Framework. AstraZeneca is a global, science-led biopharmaceutical company that focuses on the discovery, development and commercialisation of prescription medicines in Oncology and Bio-Pharmaceuticals, including Cardiovascular, Renal & Metabolism, and Respiratory & Immunology. (Photo: VietnamPlus)
AstraZeneca has been working with the vaccine-sharing scheme COVAX to begin supplying the vaccine around the world, with the majority going to low and middle-income countries as quickly as possible. In the first half of 2021, it is hoped that more than 300 million doses of the vaccine will be made available to 145 countries through COVAX, pending supply and operational challenges. These doses will be allocated equitably according to the COVAX Allocation Framework. AstraZeneca is a global, science-led biopharmaceutical company that focuses on the discovery, development and commercialisation of prescription medicines in Oncology and Bio-Pharmaceuticals, including Cardiovascular, Renal & Metabolism, and Respiratory & Immunology. (Photo: VietnamPlus)  The Government of Vietnam has declared the COVID-19 vaccines (supplied via COVAX) will be given free of charge to the population under the National Expanded Immunisation Programme. Under the country’s vaccine rollout plan, 11 priority groups will receive the first jabs – medical workers; people directly involved anti-pandemic efforts (COVID-19 prevention and control steering committees of all levels, quarantine facility staff, reporters, etc.); diplomats, customs officers and people working entry and exit procedures; military personnel; public security forces; teachers; elders above 65 years old; essential service workers (aviation, transport, tourism staff, utility workers, etc.); people with chronic health issues; people who want to study or work overseas; and people in virus-hit regions. (Photo: VietnamPlus)
The Government of Vietnam has declared the COVID-19 vaccines (supplied via COVAX) will be given free of charge to the population under the National Expanded Immunisation Programme. Under the country’s vaccine rollout plan, 11 priority groups will receive the first jabs – medical workers; people directly involved anti-pandemic efforts (COVID-19 prevention and control steering committees of all levels, quarantine facility staff, reporters, etc.); diplomats, customs officers and people working entry and exit procedures; military personnel; public security forces; teachers; elders above 65 years old; essential service workers (aviation, transport, tourism staff, utility workers, etc.); people with chronic health issues; people who want to study or work overseas; and people in virus-hit regions. (Photo: VietnamPlus)  In Hai Duong, 50 medical workers at Hai Duong city’s medical centre, and 30 others at Kim Thanh district’s will be the first in the province to be injected. The Ministry of Health has allocated the first batch of the vaccine to 13 cities and provinces nationwide, along with the Ministry of National Defence, the Ministry of Public Security and 21 hospitals. Among the localities, all having reported COVID-19 cases since January 27, the Hanoi Centre for Disease Control (CDC) is given 8,000 doses, Hai Duong CDC 32,000, and HCM City CDC 8,000. Meanwhile, the Ministry of National Defence and the Ministry of Public Security each receive 30,000 doses. (Photo: VietnamPlus)
In Hai Duong, 50 medical workers at Hai Duong city’s medical centre, and 30 others at Kim Thanh district’s will be the first in the province to be injected. The Ministry of Health has allocated the first batch of the vaccine to 13 cities and provinces nationwide, along with the Ministry of National Defence, the Ministry of Public Security and 21 hospitals. Among the localities, all having reported COVID-19 cases since January 27, the Hanoi Centre for Disease Control (CDC) is given 8,000 doses, Hai Duong CDC 32,000, and HCM City CDC 8,000. Meanwhile, the Ministry of National Defence and the Ministry of Public Security each receive 30,000 doses. (Photo: VietnamPlus)  The Ministry of Health will roll out the vaccination in all COVID-19 treatment hospitals, prioritising those working at the frontline of the fight against the coronavirus, such as healthcare workers and contact tracers. People getting the shots will be monitored via digital health records and receive e-certificates for their completion of inoculation. Vietnam aims to secure about 150 million doses of COVID-19 vaccines to achieve herd immunity. The country is also banking on homegrown COVID-19 vaccines, not only to serve the domestic demands but also to export the surplus to other countries. The frontrunner among the three currently being developed in the country, Nano Covax by Nanogen Pharmaceutical Biotechnology, is undergoing second phase of human trials, with results expected in May. (Photo: VietnamPlus)
The Ministry of Health will roll out the vaccination in all COVID-19 treatment hospitals, prioritising those working at the frontline of the fight against the coronavirus, such as healthcare workers and contact tracers. People getting the shots will be monitored via digital health records and receive e-certificates for their completion of inoculation. Vietnam aims to secure about 150 million doses of COVID-19 vaccines to achieve herd immunity. The country is also banking on homegrown COVID-19 vaccines, not only to serve the domestic demands but also to export the surplus to other countries. The frontrunner among the three currently being developed in the country, Nano Covax by Nanogen Pharmaceutical Biotechnology, is undergoing second phase of human trials, with results expected in May. (Photo: VietnamPlus)  The Ministry of Health has recommended people to receive two jabs to be fully immunised against COVID-19 with an interval of up to 12 weeks between doses. The ministry has allocated the first batch of the AstraZeneca vaccine to 13 cities and provinces nationwide, along with the Ministry of National Defence, the Ministry of Public Security and 21 hospitals. Among the localities, all having reported COVID-19 cases since January 27, the Hanoi Centre for Disease Control (CDC) is given 8,000 doses, Hai Duong CDC 32,000, and HCM City CDC 8,000. Meanwhile, the Ministry of National Defence and the Ministry of Public Security each receive 30,000 doses. (Photo: VietnamPlus)
The Ministry of Health has recommended people to receive two jabs to be fully immunised against COVID-19 with an interval of up to 12 weeks between doses. The ministry has allocated the first batch of the AstraZeneca vaccine to 13 cities and provinces nationwide, along with the Ministry of National Defence, the Ministry of Public Security and 21 hospitals. Among the localities, all having reported COVID-19 cases since January 27, the Hanoi Centre for Disease Control (CDC) is given 8,000 doses, Hai Duong CDC 32,000, and HCM City CDC 8,000. Meanwhile, the Ministry of National Defence and the Ministry of Public Security each receive 30,000 doses. (Photo: VietnamPlus)  Vietnam aims to secure about 150 million doses of COVID-19 vaccines to achieve herd immunity. The country is also banking on homegrown COVID-19 vaccines, not only to serve the domestic demands but also to export the surplus to other countries. The frontrunner among the three currently being developed in the country, Nano Covax by Nanogen Pharmaceutical Biotechnology, is undergoing second phase of human trials, with results expected in May. The third and final phase of human trials is scheduled to take place around May to September, and registration for circulation will be pushed forward to September, three months earlier than planned. (Photo: VietnamPlus)
Vietnam aims to secure about 150 million doses of COVID-19 vaccines to achieve herd immunity. The country is also banking on homegrown COVID-19 vaccines, not only to serve the domestic demands but also to export the surplus to other countries. The frontrunner among the three currently being developed in the country, Nano Covax by Nanogen Pharmaceutical Biotechnology, is undergoing second phase of human trials, with results expected in May. The third and final phase of human trials is scheduled to take place around May to September, and registration for circulation will be pushed forward to September, three months earlier than planned. (Photo: VietnamPlus)  Under the country’s vaccine rollout plan, 11 priority groups will receive the first jabs – medical workers; people directly involved anti-pandemic efforts (COVID-19 prevention and control steering committees of all levels, quarantine facility staff, reporters, etc.); diplomats, customs officers and people working entry and exit procedures; military personnel; public security forces; teachers; elders above 65 years old; essential service workers (aviation, transport, tourism staff, utility workers, etc.); people with chronic health issues; people who want to study or work overseas; and people in virus-hit regions. The priority ranking is evaluated based on criteria such as areas where COVID-19 cases are present, areas with COVID-19 deaths occurred, major cities with high population density and localities considered traffic and transit hubs. (Photo: VietnamPlus)
Under the country’s vaccine rollout plan, 11 priority groups will receive the first jabs – medical workers; people directly involved anti-pandemic efforts (COVID-19 prevention and control steering committees of all levels, quarantine facility staff, reporters, etc.); diplomats, customs officers and people working entry and exit procedures; military personnel; public security forces; teachers; elders above 65 years old; essential service workers (aviation, transport, tourism staff, utility workers, etc.); people with chronic health issues; people who want to study or work overseas; and people in virus-hit regions. The priority ranking is evaluated based on criteria such as areas where COVID-19 cases are present, areas with COVID-19 deaths occurred, major cities with high population density and localities considered traffic and transit hubs. (Photo: VietnamPlus)  Vietnam expects to receive an additional 1.3 million COVID-19 vaccine doses via COVAX Facility this month. The country is also banking on homegrown COVID-19 vaccines, not only to serve the domestic demands but also to export the surplus to other countries. The frontrunner among the three currently being developed in the country, Nano Covax by Nanogen Pharmaceutical Biotechnology, is undergoing second phase of human trials, with results expected in May. The third and final phase of human trials is scheduled to take place around May to September, and registration for circulation will be pushed forward to September, three months earlier than planned. (Photo: VietnamPlus)
Vietnam expects to receive an additional 1.3 million COVID-19 vaccine doses via COVAX Facility this month. The country is also banking on homegrown COVID-19 vaccines, not only to serve the domestic demands but also to export the surplus to other countries. The frontrunner among the three currently being developed in the country, Nano Covax by Nanogen Pharmaceutical Biotechnology, is undergoing second phase of human trials, with results expected in May. The third and final phase of human trials is scheduled to take place around May to September, and registration for circulation will be pushed forward to September, three months earlier than planned. (Photo: VietnamPlus) VNA