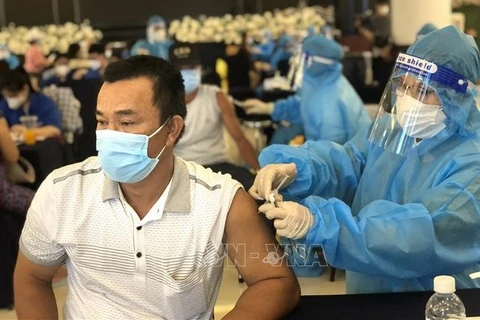
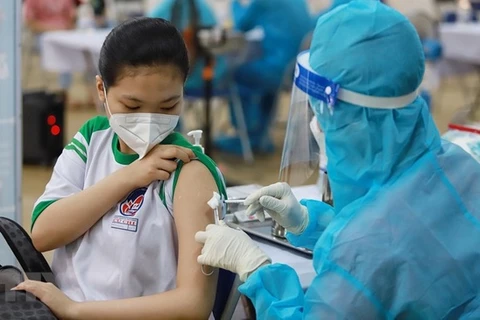
Hanoi (VNA) – The Ministry of Health on November 10 granted conditional approval of Covaxin COVID-19 vaccine produced by India's Bharat Biotech International Limited.
The vaccine is formulated from an inactivated SARS-CoV-2 antigen and is presented in single dose vials and multidose vials of 5, 10 and 20 doses.
The ministry also gave detailed conditions going along with the approval of the vaccine for emergency use in COVID-19 prevention and control.
Earlier, the World Health Organisation (WHO) issued an emergency use listing (EUL) for Covaxin developed by Bharat Biotech, adding to a growing portfolio of vaccines validated by WHO for the prevention of COVID-19 caused by SARS-CoV-2.
WHO’s EUL procedure assesses the quality, safety and efficacy of COVID-19 vaccines and is a prerequisite for COVAX vaccine supply. It also allows countries to expedite their own regulatory approval to import and administer COVID-19 vaccines.
So far, Vietnam has approved nine COVID-19 vaccines namely AstraZeneca, SputnikV, Janssen, Spikevax, Comirnaty, Vero Cell, Hayat – Vax, Abdala, and Covaxin./.
The vaccine is formulated from an inactivated SARS-CoV-2 antigen and is presented in single dose vials and multidose vials of 5, 10 and 20 doses.
The ministry also gave detailed conditions going along with the approval of the vaccine for emergency use in COVID-19 prevention and control.
Earlier, the World Health Organisation (WHO) issued an emergency use listing (EUL) for Covaxin developed by Bharat Biotech, adding to a growing portfolio of vaccines validated by WHO for the prevention of COVID-19 caused by SARS-CoV-2.
WHO’s EUL procedure assesses the quality, safety and efficacy of COVID-19 vaccines and is a prerequisite for COVAX vaccine supply. It also allows countries to expedite their own regulatory approval to import and administer COVID-19 vaccines.
So far, Vietnam has approved nine COVID-19 vaccines namely AstraZeneca, SputnikV, Janssen, Spikevax, Comirnaty, Vero Cell, Hayat – Vax, Abdala, and Covaxin./.
VNA