Hanoi (VNA) - The Ministry of Agriculture and Rural Development (MARD) on July 24 issued a document guiding the use of vaccines against African swine fever (ASF) nationwide.
The document sent to provincial and city People’s Committees said that localities can use the vaccines based on the local disease situation, and will evaluate the effectiveness following the vaccination.
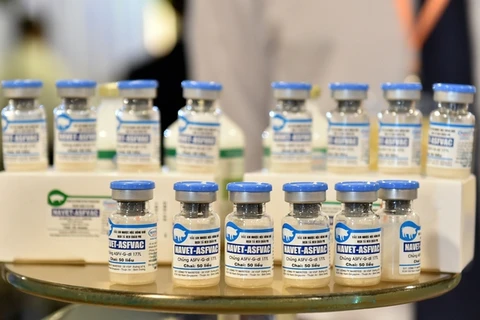
The two vaccines that will be used are NAVET-ASFVAC and AVAC ASF LIVE, which have been approved for circulation.
These are the first vaccines of their type that have received the approval. In over 100 years, there had not been a licensed commercial vaccine against ASF in the world.
Prior to the approval, a pilot programme was conducted with over 600,000 vaccine shots administered in farms across Vietnam.
Results in July 2023 show that pigs receiving the vaccine are in good health. On average, more than 95% of the vaccinated pigs have a high antibody response.
An independent assessment by a USDA (US Department of Agriculture) delegation conducted in April-May 2023 also confirmed the results of vaccine research and production from the Vietnamese side.
The evaluation shows that the Vietnam-made ASF vaccines meet international standards, including those that have been developed by scientists and submitted to the World Organisation for Animal Health (WOAH).
The MARD’s document also noted that during this vaccination drive, local pig herds might have been infected with ASF or other diseases without showing clinical symptoms.
Therefore, vaccination may cause an outbreak that leads to death. In this case, the infected must be culled according to regulations.
Local authorities will also work with relevant units and the two vaccine manufacturers, NAVETCO and AVAC Vietnam to administer the vaccines according to the manufacturer’s instruction.
Localities will also report on the vaccination results and possible bottlenecks to the MARD, through its Department of Animal Health for solutions.
The two vaccine manufacturers are also required to ensure production accordingly to the demands in the country and abroad to ensure quality and quantity as per regulations. Response plans must also be ready in case of unexpected risks that occur during the vaccination drive.
They will also provide guidelines to localities on technical criteria and the selection of pigs for vaccination.
The vaccine companies will also need to send vaccine samples to the National Center for Veterinary Drugs and Bio-Products Control No 1 for quality checks for at least 10 consecutive batches of vaccines.
The Department of Animal Health will provide support to localities and businesses in vaccine use and quality control, as well as coordination with US experts on vaccine development for different types of pigs of different ages, as well as effectiveness evaluation./.
The document sent to provincial and city People’s Committees said that localities can use the vaccines based on the local disease situation, and will evaluate the effectiveness following the vaccination.
The two vaccines that will be used are NAVET-ASFVAC and AVAC ASF LIVE, which have been approved for circulation.
These are the first vaccines of their type that have received the approval. In over 100 years, there had not been a licensed commercial vaccine against ASF in the world.
Prior to the approval, a pilot programme was conducted with over 600,000 vaccine shots administered in farms across Vietnam.
Results in July 2023 show that pigs receiving the vaccine are in good health. On average, more than 95% of the vaccinated pigs have a high antibody response.
An independent assessment by a USDA (US Department of Agriculture) delegation conducted in April-May 2023 also confirmed the results of vaccine research and production from the Vietnamese side.
The evaluation shows that the Vietnam-made ASF vaccines meet international standards, including those that have been developed by scientists and submitted to the World Organisation for Animal Health (WOAH).
The MARD’s document also noted that during this vaccination drive, local pig herds might have been infected with ASF or other diseases without showing clinical symptoms.
Therefore, vaccination may cause an outbreak that leads to death. In this case, the infected must be culled according to regulations.
Local authorities will also work with relevant units and the two vaccine manufacturers, NAVETCO and AVAC Vietnam to administer the vaccines according to the manufacturer’s instruction.
Localities will also report on the vaccination results and possible bottlenecks to the MARD, through its Department of Animal Health for solutions.
The two vaccine manufacturers are also required to ensure production accordingly to the demands in the country and abroad to ensure quality and quantity as per regulations. Response plans must also be ready in case of unexpected risks that occur during the vaccination drive.
They will also provide guidelines to localities on technical criteria and the selection of pigs for vaccination.
The vaccine companies will also need to send vaccine samples to the National Center for Veterinary Drugs and Bio-Products Control No 1 for quality checks for at least 10 consecutive batches of vaccines.
The Department of Animal Health will provide support to localities and businesses in vaccine use and quality control, as well as coordination with US experts on vaccine development for different types of pigs of different ages, as well as effectiveness evaluation./.
VNA