Vietnam authorizes commercial circulation of African swine fever vaccine
 Vietnam is the first country in the world to successfully research and produce the African swine fever (ASF) vaccine and license it for commercial circulation. It is the NAVET-ASFVAC vaccine made by NAVETCO Company.
Vietnam is the first country in the world to successfully research and produce the African swine fever (ASF) vaccine and license it for commercial circulation. It is the NAVET-ASFVAC vaccine made by NAVETCO Company.Hanoi (VNA) - African swine fever has a mortality rate as high as 100% in pigs, becoming a constant concern for the livestock industry. After more than 2 years of research, Vietnam has successfully produced a vaccine to counter the infectious disease, meeting the expectations of millions of farmers.
First commercially available vaccine
African swine fever first infected Vietnam in February 2019, then spread to the whole country. The disease has forced the destruction of over 6 million pigs, a loss of over VND 30,000 billion, directly affecting the CPI in 2020.
Up to now, the disease still infests many communities across the country. The risk of African swine fever continues to rise and spread without an effective vaccine.
Deputy Minister of Agriculture and Rural Development Phung Duc Tien said that since ASF appeared in Vietnam, the Politburo, the Party Secretariat, National Assembly, Government, and especially the Prime Minister, have undertaken drastic efforts to fight the disease and support research to produce preventative vaccines.
“The Ministry of Agriculture and Rural Development has directed the Department of Animal Health and businesses with potential, resources and experience such as NAVETCO Central Veterinary Medicine Joint Stock Company, AVAC Company and Dabaco Company to coordinate with scientists from the US Department of Agriculture receive vaccine virus varieties, technology and research, manufacture, and register for circulation of vaccines,” said Deputy Minister Phung Duc Tien.
After more than 2 years of research, NAVETCO's NAVET-ASFVAC vaccine was produced, becoming the first vaccine of its kind allowed for commercial circulation in Vietnam and in the world. This is a historic event, recognizing the efforts of the veterinary industry, businesses and Vietnamese scientists who have worked closely and effectively with scientists around the world to successfully produce a vaccine against the disease.
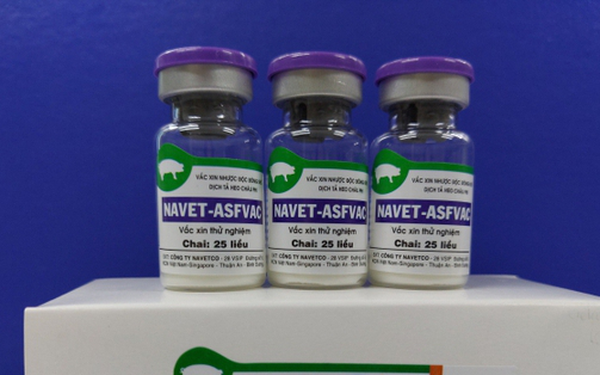
 Granting a commercial circulation license for the NAVET-ASFVAC vaccine of NAVETCO Company. (Photo: Vietnamplus)
Granting a commercial circulation license for the NAVET-ASFVAC vaccine of NAVETCO Company. (Photo: Vietnamplus)There will be 2 more vaccines
Up to now, Vietnam has 3 enterprises with sufficient potential and serious investment to research and produce African swine fever vaccine, including NAVETCO, AVAC, and Dabaco. Navetco Company has successfully researched, and produced vaccines for circulation.
It is expected that by the end of the year, Vietnam will license two more vaccines for the circulation to serve the prevention and control of African swine fever, bringing the total number of vaccines to three.
Tran Xuan Hanh, Deputy General Director of NAVETCO Company, said that the company has a capacity of producing some 50 million doses of vaccine a year. In the first phase, the company will prioritize domestic demand, and then look at opportunities for export.
"Countries really need vaccines, but when exporting we have to work with partners to test our vaccine first, and this takes time. It is expected that after 1-2 years, we will work with foreign partners to export,” said Mr. Tran Xuan Hanh.
Minister of Agriculture and Rural Development Le Minh Hoan said that for 1.7 million small households raising pigs, a pig is a valuable asset. Therefore, they will be very excited and looking forward to the achievements of Vietnamese scientists.
Minister Le Minh Hoan noted that when commercializing veterinary products such as African swine fever vaccine, in addition to the goal of profit maximization, producers should take into account social responsibility, balance between profit and cost to make it easier for people to access. This will help the livestock industry to develop more stably, he said./.